Ethics & Integrity
Ethics are moral principles that define what is right and wrong, guiding our behaviour. In research, they appear as rules or codes of conduct. Integrity means consistently applying these principles, staying true to positive values.
General principles and ethos of ethics and integrity
-
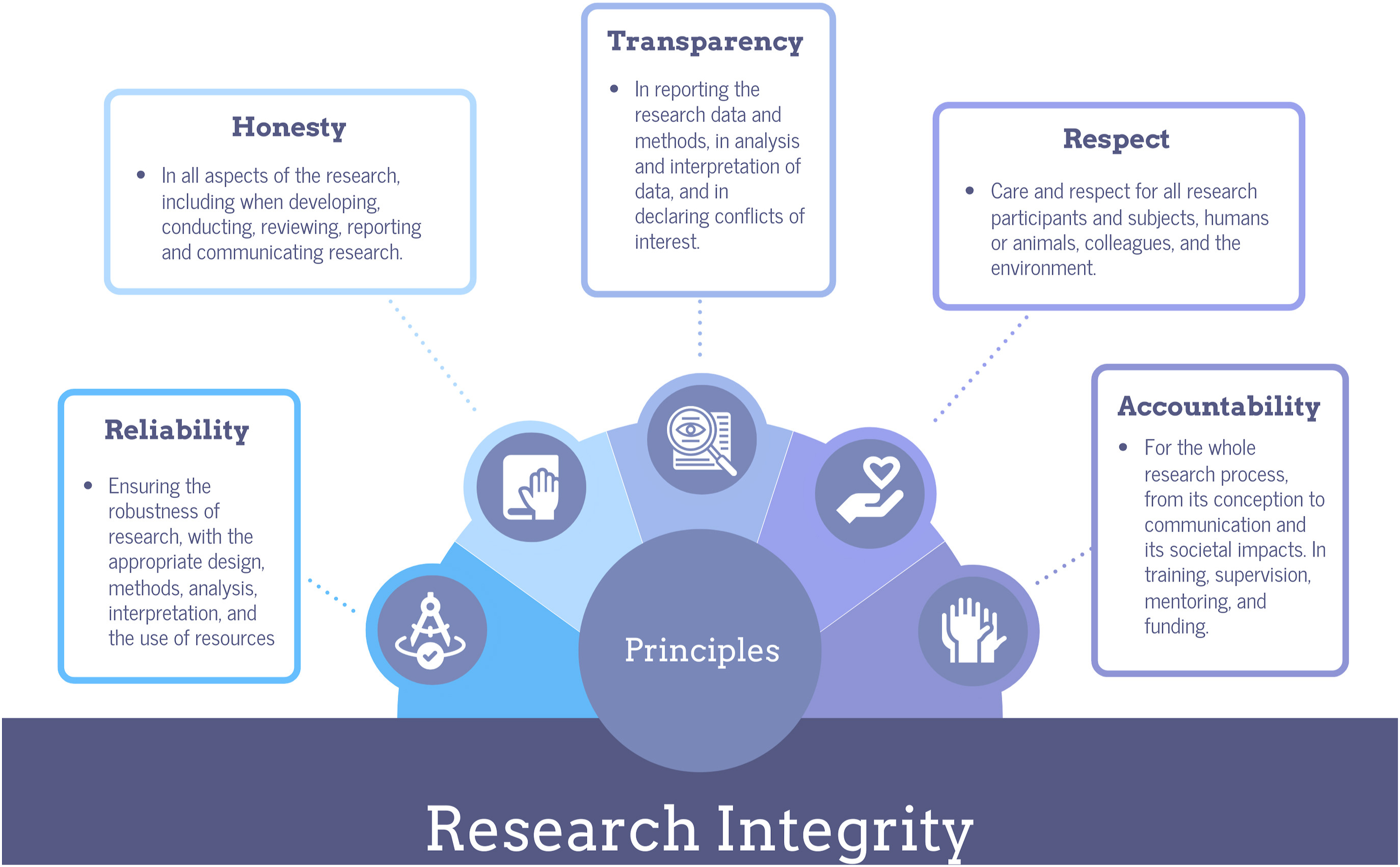
Ensure you thoroughly evaluate the ethics of your research, guided by key principles such as respect for research subjects, adherence to regulations, appropriate use of resources, research integrity, personal responsibility, accountability and sustainability – including environmental impact.
-
Be aware of all regulations impacting your work (whether imposed by the University, funders, regulatory bodies, etc.), and always adhere to these.
-
Make sure you understand the importance of Equality, Diversity, and Inclusion (EDI) considerations and how you can incorporate these principles into your work or workplace.
In line with principles of transparency and fairness, always acknowledge anyone who has contributed to your work and properly state their contributions. Use the Contributor Role Taxonomy (CRediT).
For published work, ensure all authors get a chance to approve of the work before submission, and are kept up to date on progress.
- Unviersity of Edinburgh Research Ethics Policy
- Equality, Diversity and Inclusion for students
- IAD training on Research Ethics and Integrity
- CMVM Authorship Guidance
- Contributor Role Taxonomy (CRediT)
- Good Conduct in Authorship and Publication Practice
- University of Edinburgh Fair Authorship Guidance
Determine whether your work requires specific ethical approval or considerations
-
Ethical approval is required for the use of animal or human subjects, or for the use of sensitive or personal human data. If you are working with human participants, you will also need a project sponsor – at the University of Edinburgh, this is typically ACCORD.
-
Be aware of any restrictions around the type of information you plan to collect and plan accordingly.
-
Specific restrictions around ethics may need to be considered at different stages of your work (planning, data collection and analysis, data sharing). Keep track of these at all stages and adapt to them as you progress through your research project.
For work requiring ethical approval, your line manager or supervisor should be your first port of call, but you may need to have input from your thesis committee, other research staff, your department heads, or external bodies such as research funders.

-
Important resources:
-
For research using human subjects: clinical trial regulations, human research ethics committees, ACCORD, funder regulation and guidelines.
-
For human research, understand the importance of informed consent from all research participants and ensure this is applied.
-
For research using animal subjects: refer to guidance from animal unit staff, bodies such as the National Centre for the Replacement, Refinement, & Reduction of Animals in Research (NC3Rs), Home Office licensing and the Animal (Scientific Procedures) Act 1986.
-
For research using personal or sensitive data: the Data Protection Act (DPA), General Data Protection Regulation (GDPR).
-
- Human participants in research (UKRI)
- Research Ethics support (UoE, CMVM)
- The National Centre for the Replacement, Refinement & Reduction of Animals in Research (NC3Rs)
- UK Government advice on research and testing using animals
- University of Edinburgh Data Protection
- Working with sensitive data (UoE library guidance)
If things go wrong
-
Remember that it is likely during a research project or research degree that something unexpected will happen or that something will go wrong – this is entirely normal, as are ensuing changes and adaptations to plans or procedures.
-
Try to cultivate resilience in face of these challenges, to make sure that you can withstand difficult situations and recover from them more quickly, as well as learning from mistakes or setbacks.
-
Some challenges, particularly interpersonal difficulties with colleagues and/or supervisors might be more delicate and require support and/or following specific procedures to reach a resolution. Consider which individuals you could reach out to for help (e.g., your supervisor or line manager, or thesis committee).
-
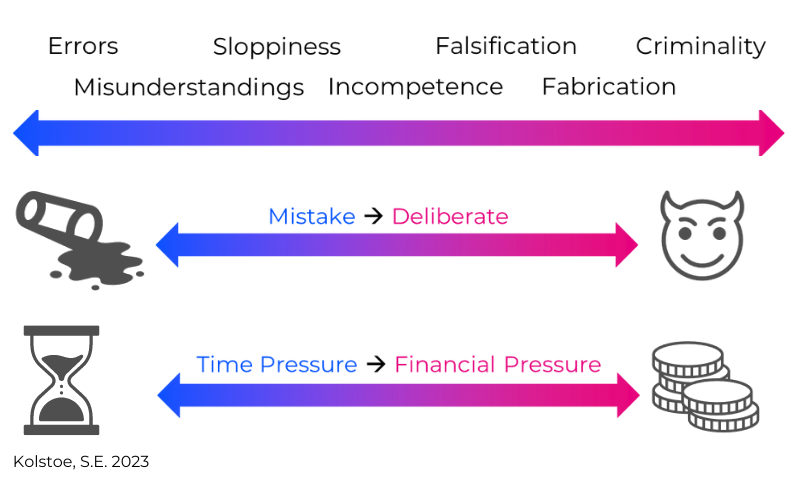
Another challenge that can occur is when research misconduct takes place.
-
Research misconduct refers to actions that threaten research integrity.
-
Misconduct can be unintentional, and not necessarily a deliberate breach of research integrity, and can be of varying degrees of severity.
-
Misconduct can include data falsification or fabrication, plagiarism, misrepresentation of findings, breach of duty of care, or a failure to meet legal, ethical, or professional obligations.
-
-
Take steps to ensure that you avoid (intentionally or unintentionally) engaging in research misconduct or questionable research practices, for instance ensuring that you fully understand what constitutes plagiarism or falsification.
-
In case you find that misconduct has taken place, inform yourself on how this should be reported, and whether this should be reported by yourself or instead referred to your line manager.
-
If you find yourself suffering from mental health difficulties during your project, be aware that the University of Edinburgh offers a range of counselling services for this purpose.
-
In case of more severe or prolonged mental or physical health difficulties that may impact your work, consider instead reaching out to the Disability and Learning Support Service or Occupational Health Services.
- University of Edinburgh Research Misconduct guidance
- CMVM Research Misconduct Informal Reporting Form
- IAD Good Conduct in Authorship and Publication Practice
- University of Edinburgh Code of Practice for Supervisors and Research Students
- UKRIO – questionable research practices
- University of Edinburgh Health and Wellbeing Hub